Lynparza 50mg | Lynparza 50mg Capsules
Apple Pharmaceuticals
Lynparza 50mg Capsules (Olaparib 50mg)
Lynparza is a type of targeted therapy called a PARP (oral poly (adenosine diphosphate–ribose) polymerase inhibitor)which has promising anti neoplasticaction in patients with metastatic breast cancer and a germline BRCA mutation.
Lynparza 50mg is an enzyme included in DNA repair and will act against cancers in people with hereditary BRCA1 or BRCA2 mutations, which involve some ovarian, breast and prostate cancer
Lynparza 50mg Capsules is a prescription drug which used under the supervision of medical practioners
Lynparza 50mg is an enzyme included in DNA repair and will act against cancers in people with hereditary BRCA1 or BRCA2 mutations, which involve some ovarian, breast and prostate cancer
Lynparza 50mg Capsules is a prescription drug which used under the supervision of medical practioners
INDICATION
 |
| Lynparza 50mg capsules |
Lynparza 50 capsules is used for the treatment in patients with first line maintenance BRCAm advanced ovarian cancer
Lynparza 50mg is used for the treatment in patients with maintenance recurrent ovarian cancer.
Lynparza is used for the treatment in patients withadvanced gBRCAm ovarian cancer
Lynparza is indicated for the treatment in patients with HER2 – negative metastatic breast cancer.
Lynparza 50mg is used for the treatment in patients with maintenance recurrent ovarian cancer.
Lynparza is used for the treatment in patients withadvanced gBRCAm ovarian cancer
Lynparza is indicated for the treatment in patients with HER2 – negative metastatic breast cancer.
DOSAGE
Ovarian cancer :
 |
| ovarian cancer |
Maintenance treatment of recurrent ovarian cancer
The tablet usual dose is 300mg (two 150mg – mg tablets) PO BID
Continue treatment until disease progression or unacceptable toxicity
Monotherapy for advanced BRCA – mutated ovarian cancer
The tablet usual dose is 300mg (two 150mg – mg tablets) PO BID
The capsules usual dose is 400mg (eight 50mg- mg capsules) PO BID
Continue treatment until disease progression or unacceptable toxicity.
Maintenancetreatment for advanced BRCA-mutated ovarian cancer
The tablet usual dose is 300mg (two 150mg – mg tablets) PO BID
Follow the treatment until disease progression,lynparza undesirable toxicity or completion of 2 years of treatment
Finishing of 2 years of treatment
• Patients with full response (no radiologic evidence): discontinue treatment
• Patients with indication of disease and may asset from continuous treatment: cure beyond 2 years.
MECHANISM
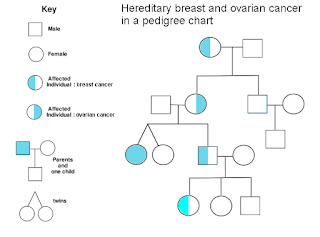 |
| ovarian cancer in a chart |
Olaparib 50mg capsules belongs to targeted therapy. Olaparib is also called as poly (ADP-ribose) polymerase (PARP) enzyme inhibitor, involving PARP1, PARP2, and PARP3. PARP enzymes are containing in DNA transcription, cell cycle regulation, and DNA repair.
Olaparib belongs to potent oral PARP inhibitor which induces synthetic fatality in BRCA 1/2 deficient tumor cells via the formation of double-stranded DNA cutsthat cannot be accurately repaired, which causesdivision of cellular homeostasis and cell death
Olaparib belongs to potent oral PARP inhibitor which induces synthetic fatality in BRCA 1/2 deficient tumor cells via the formation of double-stranded DNA cutsthat cannot be accurately repaired, which causesdivision of cellular homeostasis and cell death
ADME
Absorption: Maximum plasma concentration 1 to 3 hours
Distribution: volume of distribution 167 +/-196 L, plasma protein binding is 82%
Metabolism: Primarily metabolised by CYP3A4
Elimination: Excreted through the urine 44% and 42% through the feces.
Half – life of Lynparza 11.9 hours
Distribution: volume of distribution 167 +/-196 L, plasma protein binding is 82%
Metabolism: Primarily metabolised by CYP3A4
Elimination: Excreted through the urine 44% and 42% through the feces.
Half – life of Lynparza 11.9 hours
PRECAUTION
• Haematological toxicity will cause in patients treated with Lynparza contain diagnoses or findings of generally mild or moderate anaemia, neutropenia, thrombocytopenia and lymphopenia.
• When Lynparza monotherapy administrated to patient the incidence of myelodysplastic syndrome will occur. • On basis of mechanism of action (PARP inhibition), Lynparza could cause risk to fetal when administered to a pregnant woman.
• Lynparza combination with strong or moderate CYP3A inhibitors is not recommended.
• When Lynparza monotherapy administrated to patient the incidence of myelodysplastic syndrome will occur. • On basis of mechanism of action (PARP inhibition), Lynparza could cause risk to fetal when administered to a pregnant woman.
• Lynparza combination with strong or moderate CYP3A inhibitors is not recommended.
DRUG INTERACTION
Concomitant use with myelosuppressive anticancer agents contains DNA damaging agents, indicate a potentiation and prolongation of myelosuppressive toxicity.
Combination of strong CYP3A inhibitors (itraconazole, telithromycin, clarithromycin,) will increase Olaparib 50mg plasma concentration.
Lynparza combination with strong CYP3A inducers (itraconazole, telithromycin, clarithromycin,) will decrease Olaparib 50mg plasma concentration.
Combination of strong CYP3A inhibitors (itraconazole, telithromycin, clarithromycin,) will increase Olaparib 50mg plasma concentration.
Lynparza combination with strong CYP3A inducers (itraconazole, telithromycin, clarithromycin,) will decrease Olaparib 50mg plasma concentration.
MISSED DOSE
 |
| Lynparza 50mg capsules |
If dose is missed the have the dose immediately before next dose timing reaches or skip the missed dose and continue the regular schedule.
Consult doctors regarding missed dose.
Consult doctors regarding missed dose.
STORAGE
Store at 20℃ to 25℃
SIDE EFFECTS
 |
| side effect |
Common side effects for patients taking Olaparib :
• Decreased white blood cell count
• Abdominal pain
• Vomiting
• Upper respiratory tract infection
• Anemia
• Decreased neutrophils
• Musculoskeletal pain
• Diarrhea
• Decreased platelet count
• Decreased Haemoglobin
• Nausea
• Fatigue (including weakness)
• Increased serum creatinine
• Myalgia
• Headache
• Skin rash
• Back pain
• Taste changes
• Cough
• Swelling
• Dizziness
• Constipation
• Urinary tract infection
• Heartburn
• loss of appetite
• Shortness of breath
Comments
Post a Comment